Brindle Group
Molecular imaging (MRI and MRS)

Research summary
The aim of our laboratory is to develop imaging methods that can be used in the clinic to detect early tumour responses to treatment.
Introduction
The primary focus of our work is early detection of treatment response with the aim of developing imaging methods that could be used in early phase clinical trials to get an indication of drug efficacy, and subsequently in the clinic to guide treatment in individual patients (Brindle, Nat Rev Cancer 2008; 8: 1). We have also started to work on the more challenging problem of early detection and the development of imaging methods that potentially could be used for patient screening.
Imaging metabolism with hyperpolarised 13C-labelled cell substrates
MRI gives excellent images of soft tissues, such as tumours. The technique works by imaging the distribution and MR properties of tissue water protons, which are very abundant (60–70 M in tissues). However, we can also use MR to detect metabolites in vivo. The problem is that these molecules are present at ~10,000× lower concentration than the protons in tissue water, which makes them hard to detect and almost impossible to image, except at very low resolution. We have been collaborating with GE Healthcare in the development of a technique, termed ‘hyperpolarisation’, that increases sensitivity in the MRI experiment by more than 10,000×. With this technique we inject a hyperpolarised 13C-labelled molecule and now have sufficient sensitivity to image its distribution in the body and the distribution of the metabolites produced from it.
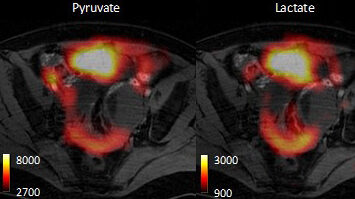
Previously we had shown we can detect early response to chemotherapeutic drugs by monitoring decreased tumour utilization of one cell metabolite, pyruvate, and then detect subsequent cell death by watching the increased metabolism of another metabolite, fumarate. We are taking these forward in a clinical trial, which started this year, to detect treatment response in breast cancer patients after a clinical device for hyperpolarising 13C-labelled pyruvate and fumarate was installed in the Department of Radiology in Addenbrooke’s Hospital last. Detecting treatment response through the decrease in hyperpolarised 13C label exchange between injected pyruvate and endogenous lactate is analogous to monitoring response through measurements of the decrease in 18fluorodeoxyglucose (18FDG) uptake using Positron Emission Tomography (PET), and indeed we have compared these two methods directly. However, both methods only probe three steps in the glycolytic pathway, which is responsible for the metabolism of glucose to lactate and which is often highly up-regulated in tumour cells. These three steps are vascular delivery, plasma membrane transport (via the glucose transporters) and hexokinase activity, in the case of 18FDG; and vascular delivery, plasma membrane transport (via the monocarboxylate transporters) and lactate dehydrogenase activity, in the case of hyperpolarized [1-13C]pyruvate. Both the enzymes and the transporters are up-regulated in tumour cells. We have shown that by using a spin echo experiment that we can monitor the extravasation and tumour cell uptake of hyperpolarised [1-13C]lactate and pyruvate (Kettunen et al., Magn. Reson. Med. 2013; 70: 1200).
A potentially better way to detect response would be to monitor flux through the entire pathway, from glucose to lactate. However, a drawback of the hyperpolarisation technique is that the polarization is relatively short-lived and hitherto we thought that it would not survive passage of the 13C label between glucose and lactate. We have now shown that deuteration of the molecule ([U-2H, U-13C]glucose) extends the lifetime sufficiently that we can detect flux of 13C label through all 10 steps of the glycolytic pathway between glucose and lactate (see Figure 1).

Figure 1: The 1H reference image is a conventional image of tissue water protons and shows the tumour (outlined in white). The 13C chemical shift images were acquired following i.v. injection of hyperpolarised [U-2H, U-13C]glucose and show glucose in the abdomen, however lactate is only present within the tumour, reflecting the high rate of tumour glycolysis and the accumulation of lactate. The images effectively demonstrate the ‘Warburg effect’. The urea image shows non-polarised urea in an adjacent tube, which was used as a standard. From: Rodrigues et al., Nat Med 2014; 20: 93
Moreover, this flux is substantially decreased in lymphoma tumours 24 h after chemotherapy (Rodrigues et al., Nat Med. 2014; 20: 93). We also have some preliminary data showing that we can detect flux of label into the pentose phosphate pathway and therefore the capacity of the tumour cells to resist oxidative stress. The ability of certain cancer cells to resist oxidative stress has been correlated with aggressiveness and drug resistance.
To combat the short lifetime of the polarisation we have been examining the possibility of ‘parking’ the polarisation in a longer-lived so-called singlet state. Although we have successfully generated this state and observed it in vivo we have yet to demonstrate a significant benefit from doing this (Marco-Rius et al., NMR Biomed 2013; 26: 1696). Polarisation is lost primarily through cross-relaxation between the hyperpolarised 13C spins in the labelled molecule and protons in either the molecule itself or in solvent water. We have demonstrated that cross relaxation with water protons could provide a method for tracking, in a proton image of the body, the passage of a bolus of the labelled material (Marco-Rius et al., Contrast Media Mol Imaging. 2014; 9(2): 182). The method avoids sampling and thus destroying the 13C polarisation and turns the inevitable loss of polarisation due to cross relaxation into a useful signal.
Future directions
Aberrant glycosylation is a hallmark of cancer. We will continue development of a novel method for assessing tumour glycosylation state, in which sugar analogues are incorporated metabolically by tumour cells in vivo and detected subsequently by a highly selective chemical reaction (‘click chemistry’) with an imaging probe (Neves et al., Bioconjug. Chem. 2013; 24: 934 (cover article); Stairs et al., Chembiochem 2013; 14: 1063; Wainman et al., Org. Biomol. Chem. 2013; 11: 7297). We are currently examining whether this method can be used to assess the metastatic potential of tumours.
We will continue development of a targeted radionuclide and fluorophore-labelled imaging agent for detecting cell death, with a view to commercial development in the preclinical arena and also translation to the clinic. We will continue with the development and application of new hyperpolarised 13C labelled metabolic tracers and have recently started the first clinical trial using pyruvate in the Department of Radiology.

Professor Kevin Brindle
Senior Group Leader
Related News
See all news-
Imaging technique allows rapid assessment of ovarian cancer subtypes and their response to treatment
6th December 2024
An MRI-based imaging technique developed at the Institute predicts the response of ovarian cancer tumours to treatment, and rapidly reveals how well treatment is working, in patient-derived cell models.
Find out more -

Imaging magnetised molecules could predict drug resistance in breast cancer patients
30th September 2020
New research suggests that a scanning technique, called carbon-13 hyperpolarised imaging, could be used to predict treatment response in breast cancer patients.
Find out more -

Prof Kevin Brindle elected as a Fellow of the Royal Society
29th April 2020
Prof Kevin Brindle has joined 50 exceptional scientists from around the world to be elected as a Fellow of the Royal Society.
Find out more