New clue discovered for why some cancer drugs fall short
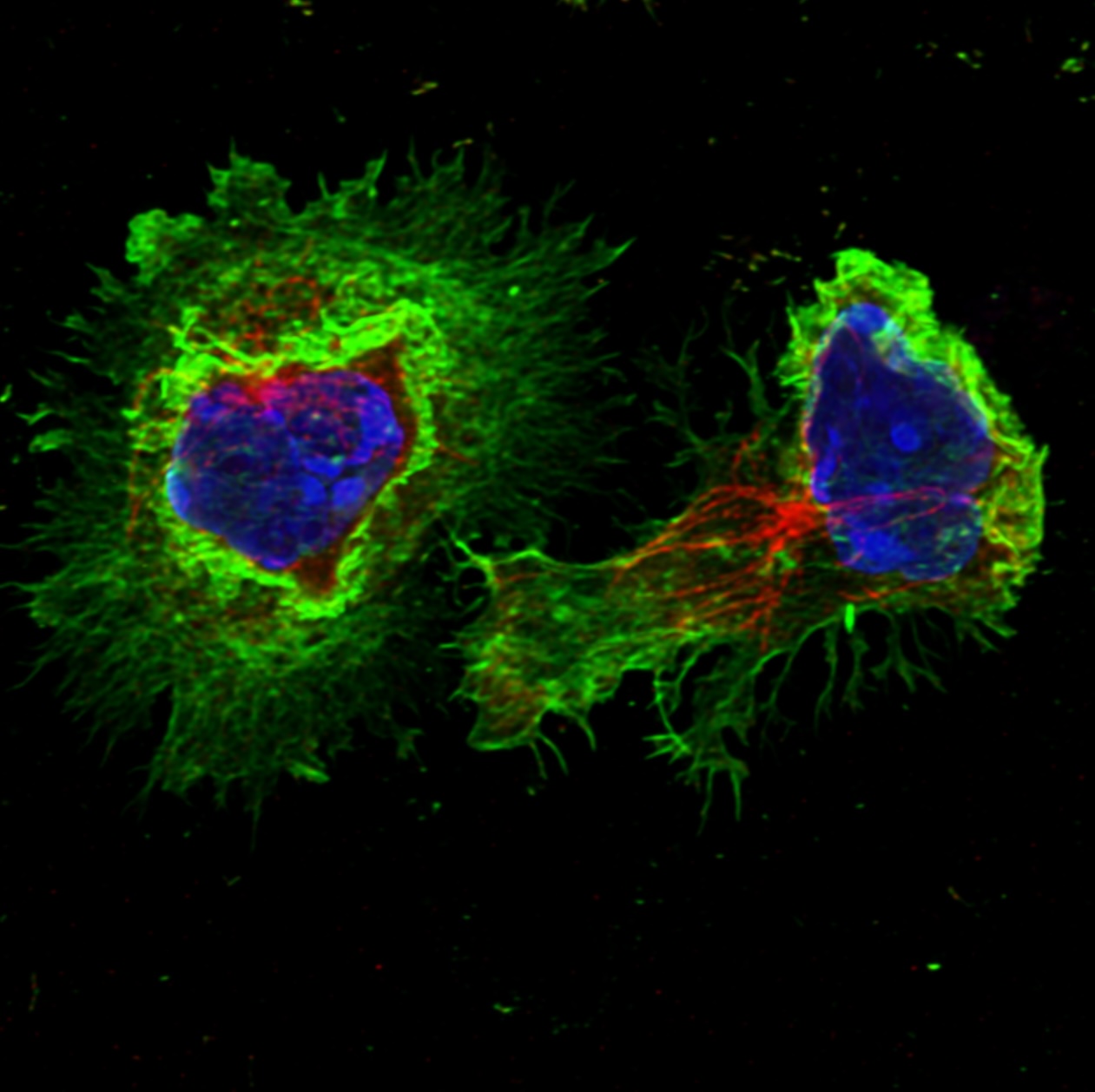
New research from the de la Roche Group has revealed that Hedgehog signalling plays a key role in controlling how immune cells migrate into tumours, a discovery that may explain why some cancer drugs have underperformed in clinical trials.
Hedgehog is a pathway cells use to communicate during early development and to help maintain tissues in the body. In some cancers, including basal cell carcinoma and medulloblastoma, the pathway becomes abnormally active and drives tumour growth. Targeted drugs that inhibit the Hedgehog pathway, including FDA-approved vismodegib and sonidegib, have improved patient outcomes in these cancers.
However, despite encouraging experimental results in laboratory settings, Hedgehog inhibitors have shown little clinical benefit for patients with other forms of the disease, such as pancreatic, prostate and colorectal cancers in which Hedgehog signalling is increased.
The new study from the de la Roche Group, published today (11 July 2025) in Science Immunology, suggests a potential explanation. The researchers found that Hedgehog inhibitors can stop the movement of cytotoxic T cells, a key type of immune cell, into tumours. These cytotoxic T cells play a critical role in the immune response against cancer by efficiently killing cancer cells, and their presence inside tumours is known to be one the strongest predictors of positive patient outcomes across many cancer types.
Importantly, the study found that this effect on T cell migration does not follow the usual Hedgehog signalling cascade. Instead, it occurs directly through a mechanism involving the SMO (Smoothened) protein, which acts as a signal hub controlling cell movement.
These findings highlight an unexpected way in which Hedgehog inhibitors may interfere with the immune response to cancer. They could help explain why clinical trials of these drugs in some solid tumours have produced mixed or disappointing results.
It also points to potential new strategies for improving how Hedgehog inhibitors are used in the clinic. These include adjusting dosing schedules to allow immune cells to infiltrate tumours or developing inhibitors that selectively block tumour-driving signals while sparing immune cell function.
This work was made possible with the support of the Biological Resource Unit, Bioinformatics, Compliance & Biobanking, Flow Cytometry, Histopathology, Microscopy, and Research Instrumentation and Cell Services core facilities at the Cancer Research UK Cambridge Institute.
Dr Chrysa Kapeni, first author said “We’ve known for a long time that Hedgehog signalling drives the growth of certain cancers. Our work has uncovered an unexpected role for the pathway, controlling how cytotoxic T cells migrate into tumours. That’s a really important piece of the puzzle, as not only does it explain why Hedgehog inhibitors haven’t performed as well in the clinic as people expected, it also opens up new possibilities for making T cell-based therapies more effective.”
“We have been fortunate to work closely with Dr Kate Fife, with whom we are running our laboratory’s clinical research study at Addenbrooke’s Hospital and are very grateful to the patients participating in the study. This collaboration showcased the clinical relevance of our previously unknown finding that Hedgehog siganlling directs how T cells move. Building on this knowledge,we are currently engineering T cells with an improved ability to infiltrate and attack solid tumours. If successful, this could pave the way for more effective CAR T cell therapies and ultimately help to improve outcomes for people with cancer.”
Dr Maike de la Roche, Group Leader at the Cancer Research UK Cambridge Institute
Related News
See all news-
1M to advance AI powered personalised ovarian cancer care
19th February 2026
Researchers from our Brenton Group are part of an international team awarded the Global Ovarian Cancer Research Consortium’s inaugural AI Accelerator Grant.
Find out more -

Professor Sir Steve Jackson elected as a fellow of the American Association for Cancer Research
16th February 2026
Senior Group Leader Sir Steve Jackson has been elected as a Fellow of the American Association for Cancer Research Academy in the Class of 2026.
Find out more -
New immune pathway offers treatment hope for childhood brain tumours
3rd February 2026
A newly discovered immune pathway could lead to gentler treatments for multiple childhood brain cancers, according to new research from our Gilbertson Group published today in Nature Genetics.
Find out more