Microscopy

Providing expertise, training and instrumentation for a range of advanced light microscopy techniques across scales and for a variety of samples.
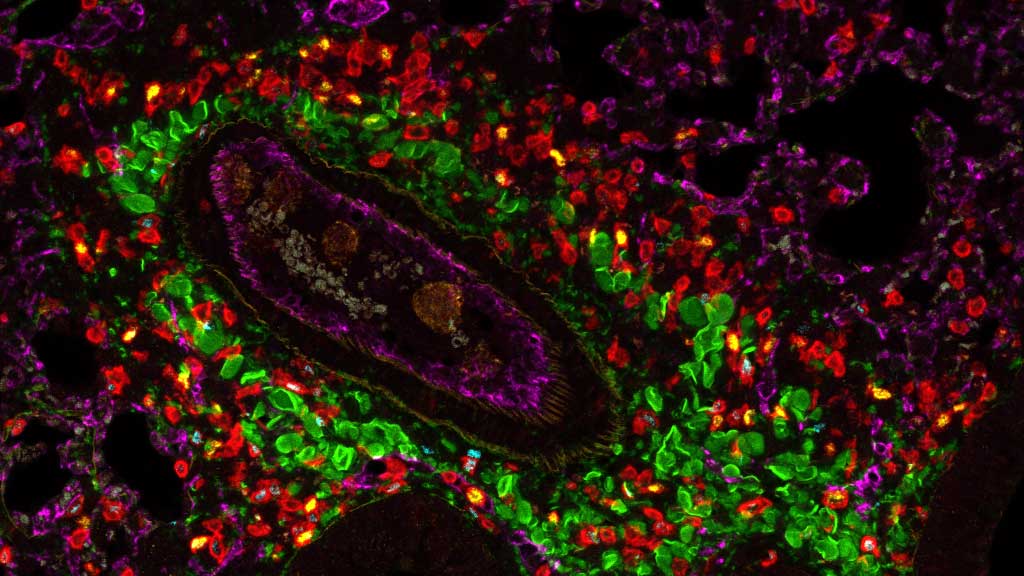
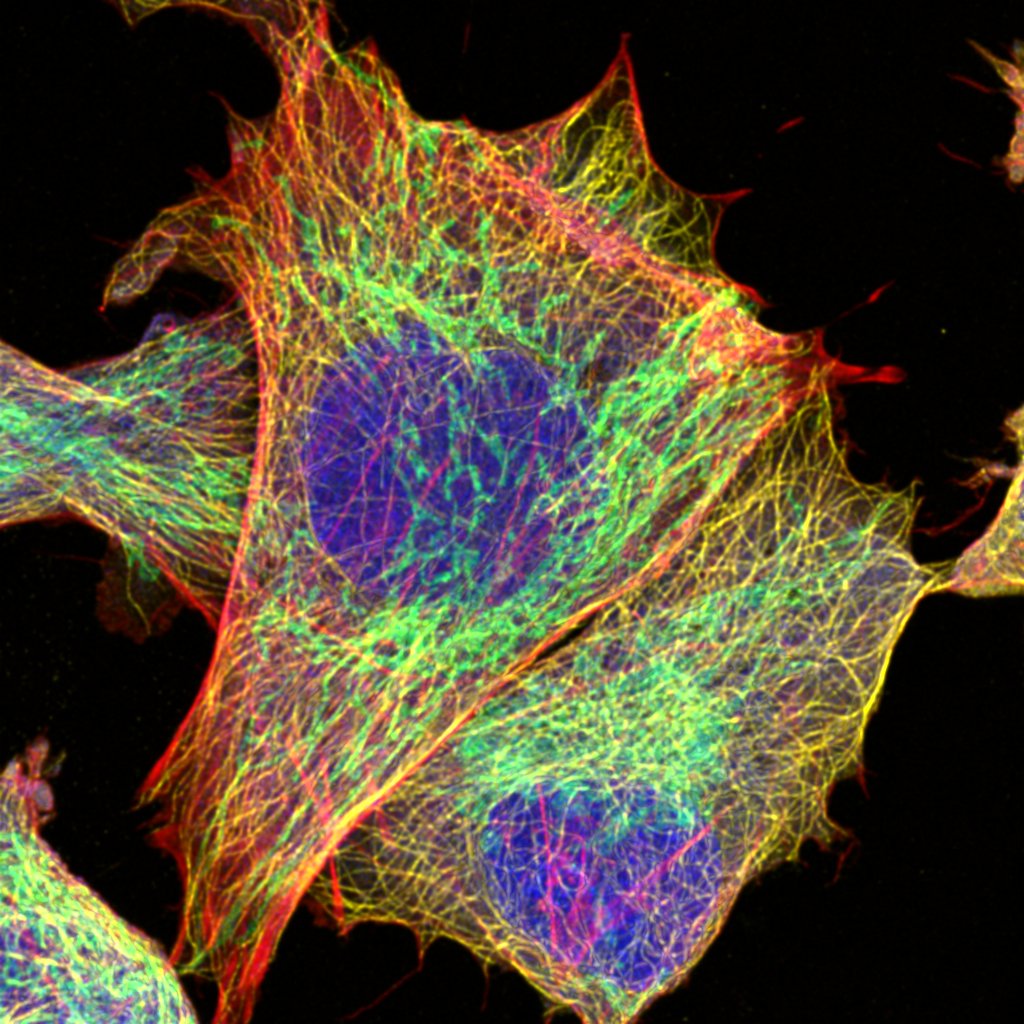
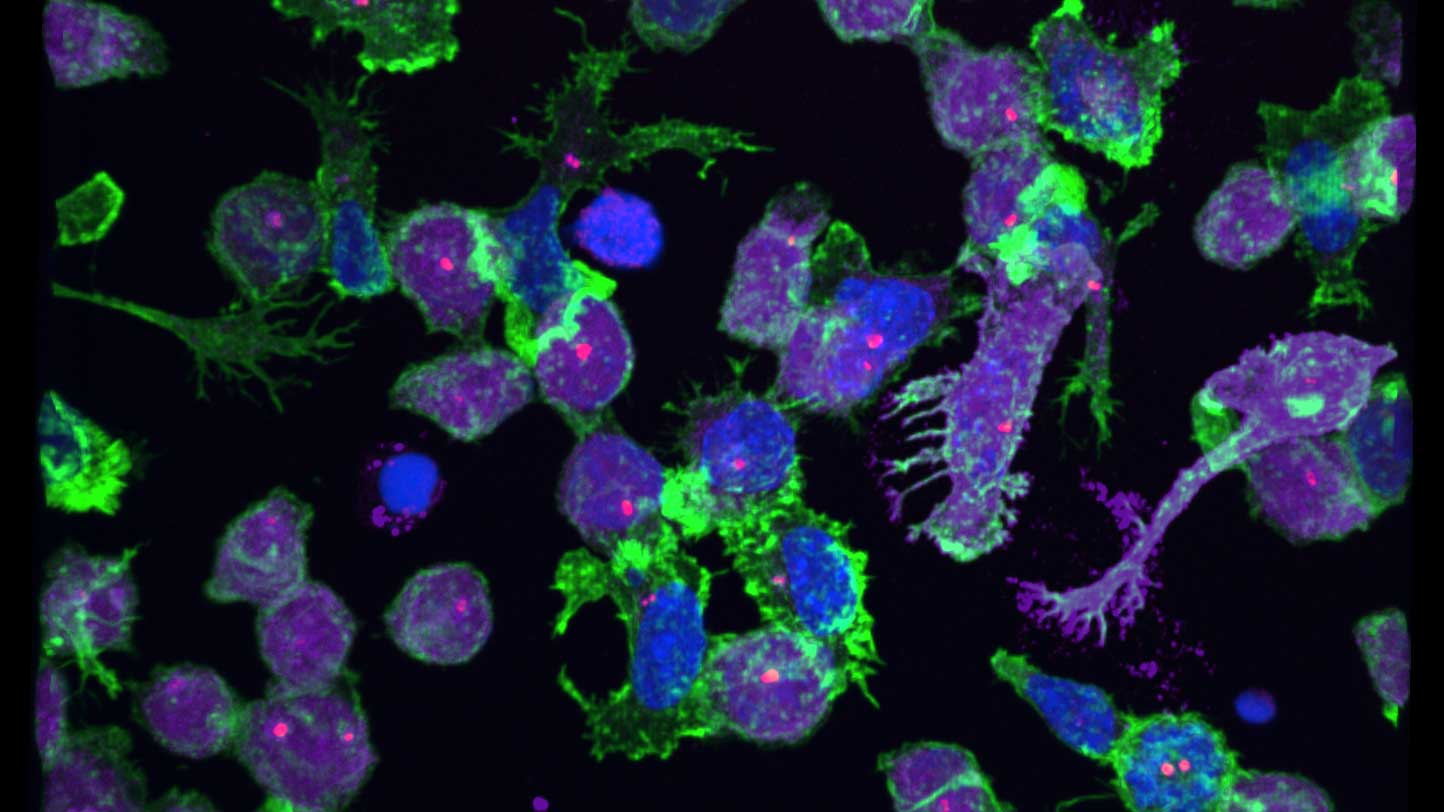
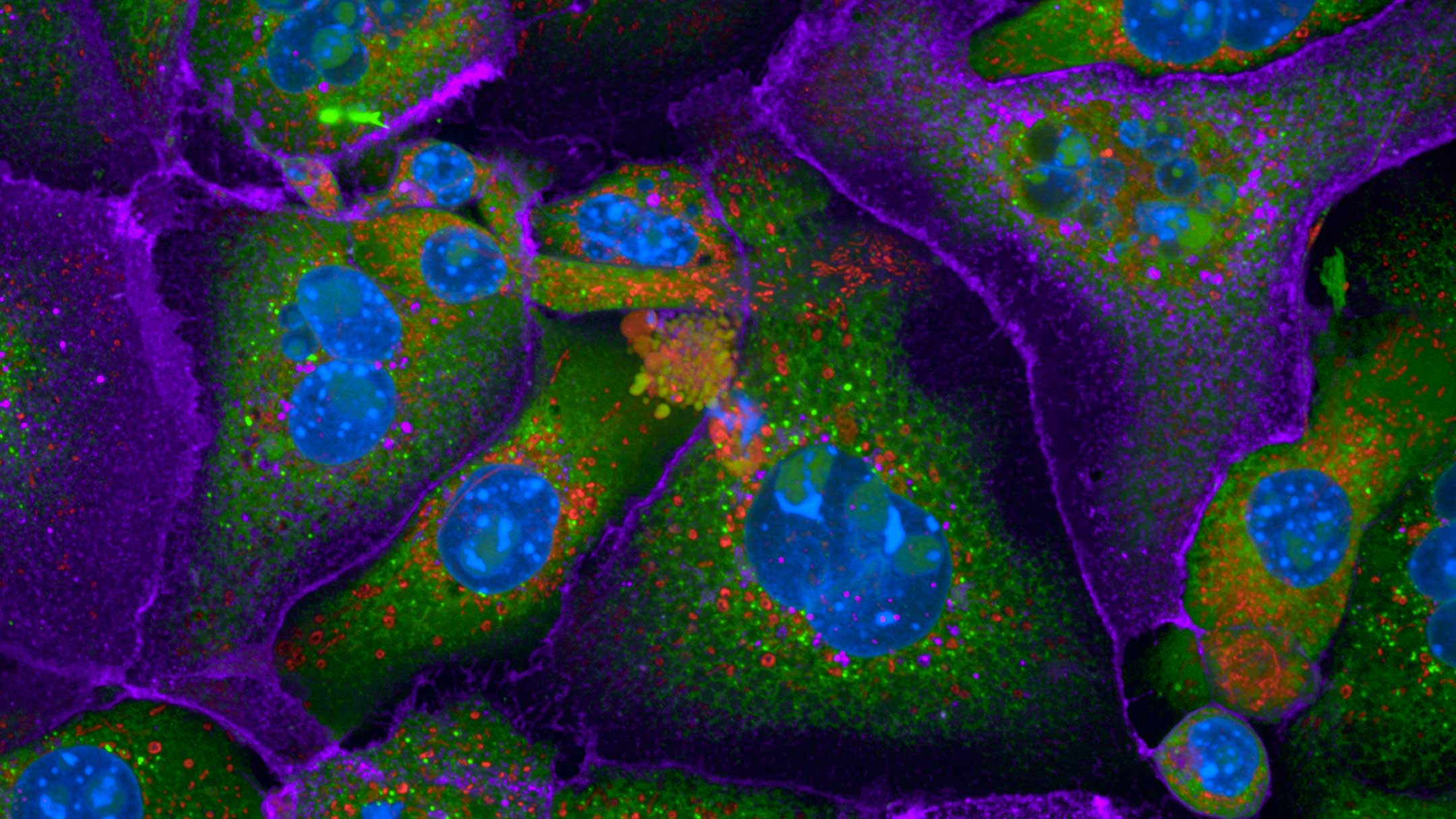
We provide training and support to scientists in a wide range of image acquisition and analysis techniques. Instruments in our facility range from wide-field to non-linear multi-photon and lifetime imaging systems for cell and tissue studies. We are specialised in the following areas of expertise:
- Advanced live-cell imaging using wide-field and spinning disc imaging systems
- Laser scanning confocal microscopy
- Quantitative high content image acquisition and analysis
- Light sheet microscopy of living and cleared samples
- STED super-resolution microscopy
- Non-linear imaging techniques such as multi-photon and second harmonic imaging
- Qualitative fluorescence life-time imaging (TauSense)

Dr Andreas Bruckbauer
Core Facility Manager
Equipment
-
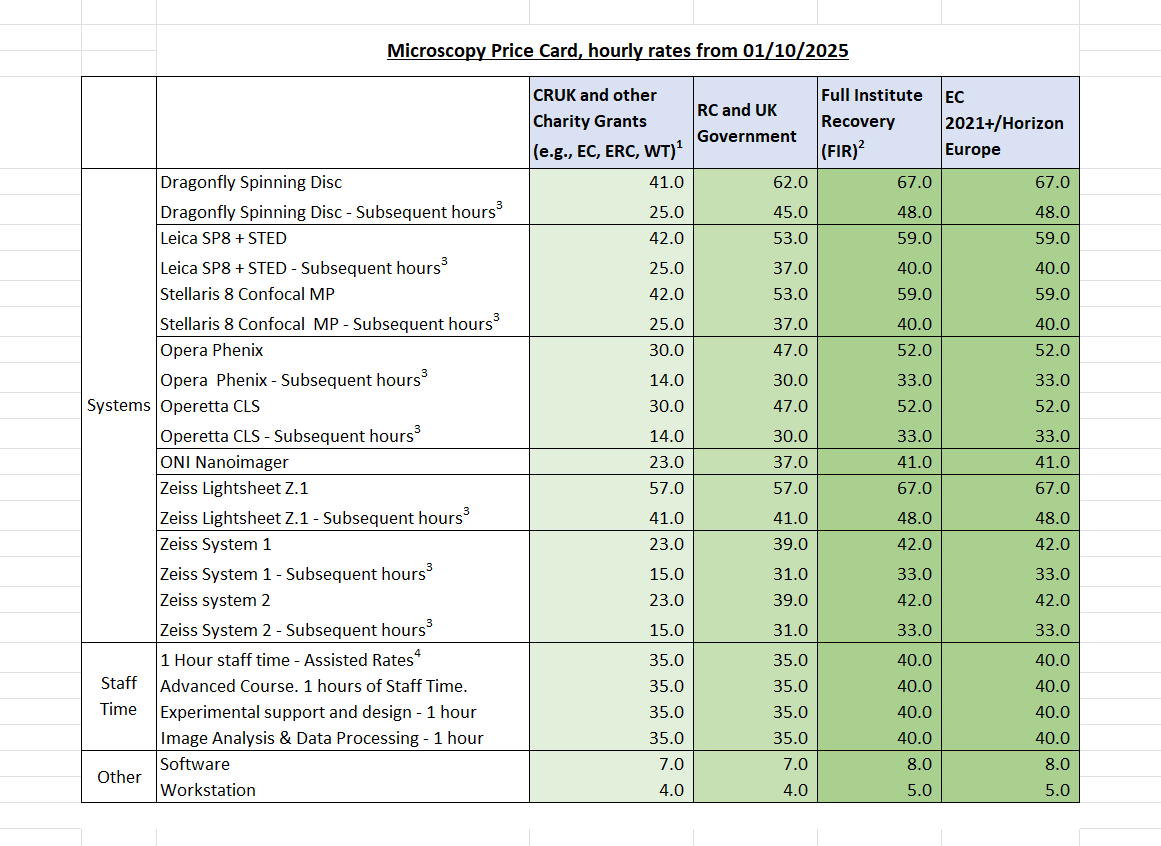
Costs and charging
The institute recovers costs from the grants and projects used in the core facilities and this is reviewed regularly. The hourly rates (in £) for the instruments and services within the core can be found here.
Related News
See all news-
New immune pathway offers treatment hope for childhood brain tumours
3rd February 2026
A newly discovered immune pathway could lead to gentler treatments for multiple childhood brain cancers, according to new research from our Gilbertson Group published today in Nature Genetics.
Find out more -
Order of cancer-driving mutations affects the chance of tumour development
3rd December 2025
New research from the Winton Group has revealed that the order of cancer-driving mutations plays an important role in whether tumours in the intestine can develop.
Find out more -
Single-cell study sheds new light on why ovarian cancer becomes resistant to chemotherapy
11th August 2025
Researchers at the Cancer Research UK Cambridge Institute and Stanford University have mapped how ovarian cancer cells respond to chemotherapy at an unprecedented level of detail, offering new insights into why treatment resistance develops.
Find out more
Laboratory Efficiency Assessment Framework (LEAF)
Microscopy contributed to the Institute’s LEAF Silver accreditation, see the Sustainability webpage for more information.