Biffi Group
Ecology of cancer

Research summary
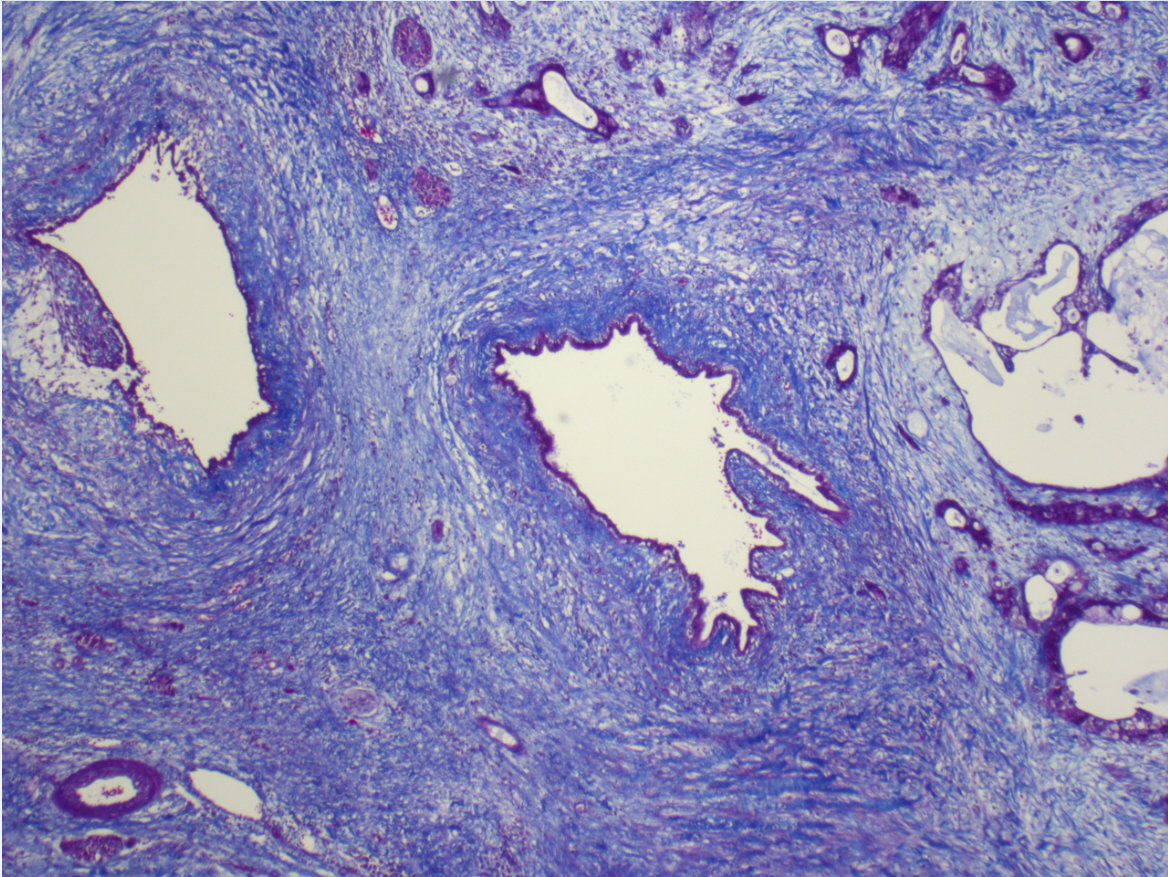
A unique feature of pancreatic cancer is the presence of many non-cancerous cells called fibroblasts, which outnumber the cancer cells. Normally, fibroblasts help maintain tissue structure, but in pancreatic cancer, they are reprogrammed by cancer cells to support cancer growth and resist treatment. By understanding how fibroblasts and cancer cells interact, we aim to develop new therapies to treat pancreatic cancer.
Introduction
Our group’s vision is to identify malignant-stromal signatures to stratify PDAC patients (e.g., based on genetics, fibroblast composition, disease stage, and age) for tailored therapies and earlier detection strategies.
Specifically, our group studies the crosstalk between cancer-associated fibroblasts (CAFs) in the tumor stroma and PDAC malignant cells. Our overarching goal is to understand how these heterogenous cell communities cooperate to drive disease progression and to apply this knowledge to develop diagnostics and therapeutics.
In particular, our research focuses on 3 PDAC-focused themes:
- Genetics and the tumour microenvironment
- Heterogeneity and functions of distinct fibroblast populations
- Systemic disease (metastases and cachexia)

Dr Giulia Biffi
Junior Group Leader
Areas of interest
Group Members
-

Giulia Biffi
Group Leader
-

Mireia Vallespinos-Serrano
Senior Scientific Associate
-

Gianluca Mucciolo
Research Associate
-

Wenlong Li
Research Associate
-

Priscilla Cheng
Postgraduate Student
-

Marta Zaccaria
Postgraduate Student
-

Joaquin Araos Henríquez
Postgraduate Student
-

Eloise Lloyd
Postgraduate Student
-

Paul Johnson
Research Assistant
-

Judhell Manansala
Research Assistant
-

Debasmita Mukherjee
Research Associate
-

Sally Mills
Scientific Associate
-

Muntadher Jihad
Bioinformatician
-

Sneha Harish
Research Assistant
Related News
See all news-

Career Foundation Fellowships from Pancreatic Cancer UK awarded to two Institute postdocs
27th February 2024
Dr Shalini Rao and Dr Gianluca Mucciolo have each received fellowships to fund research into new treatments and early detection of pancreatic cancer.
Find out more -

Cambridge researchers receive £4m from Cancer Grand Challenges to take on some of cancer’s toughest challenges
16th June 2022
The team will investigate cancer cachexia – a debilitating wasting condition people often experience in the later stages of their cancer that imparts a poor prognosis and quality of life – seeking to develop novel therapies.
Find out more -

Dr Giulia Biffi awarded UKRI Future Leader Fellowship
8th September 2021
Dr Giulia Biffi has been awarded a UK Research and Innovation’s (UKRI) Future Leader Fellowship, as one of nearly 100 of the UK’s most promising scientists and researchers.
Find out more
Further reading
-

Cancer Discovery News
Find out moreA subset of myofibroblasts promote metastasis of pancreatic cancer.
-

How cancer hijacks healthy cell functions
Find out moreInterview with the Naked Scientists.
-

CAFs and cross-talk – a recipe to close the gap on pancreatic cancer?
Read moreCancer Research UK spoke to Giulia about her passion for pancreatic cancer research and what’s next.
Publications
-
Protocol for the characterization of the pancreatic tumor microenvironment using organoid-derived mouse models and single-nuclei RNA sequencing.
E-pub date: 20 Sep 2024
-
Scribble Deficiency Promotes Pancreatic Ductal Adenocarcinoma Development and Metastasis.
E-pub date: 16 Sep 2024
-
Modelling the micro- and macro- environment of pancreatic cancer: from patients to pre-clinical models and back.
E-pub date: 1 Apr 2024
-
EGFR-activated myofibroblasts promote metastasis of pancreatic cancer.
E-pub date: 8 Jan 2024
Laboratory Efficiency Assessment Framework (LEAF)
The Biffi Group contributed to the Institute’s LEAF Silver accreditation, see the Sustainability webpage for more information.