Bioanalytical mass spectrometry

Providing capabilities to measure drug molecules in biological matrices.
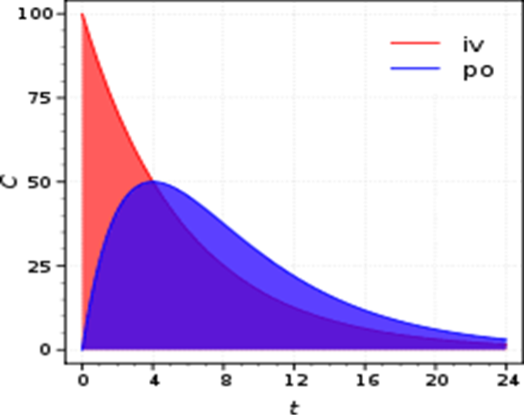
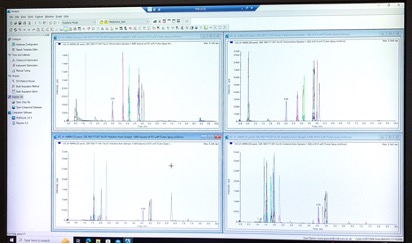
We provide bioanalytical and pharmacokinetic support for the research groups and associated clinical trials within the institute, creating a bridge between the laboratory and clinic. We offer a wide range of services, performing bioanalysis using mass spectrometry. We can quantify therapeutic drugs as well as small molecule cancer related metabolites (targeted metabolomics). We analyse samples from a wide variety of matrices, including plasma, blood, tissues, tumours, and cells.

Richard Houghton
Core Facility Manager
Services
Team members
-

Richard Houghton
Core Facility Manager
-

Maria Pawula
Principal Scientific Associate
-

Charlotte Baker
Research Associate
Laboratory Efficiency Assessment Framework (LEAF)
Bioanalytical Mass Spectrometry contributed to the Institute’s LEAF Silver accreditation, see the Sustainability webpage for more information.