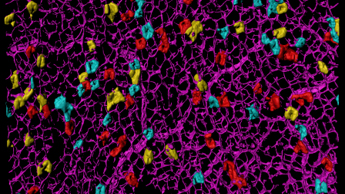
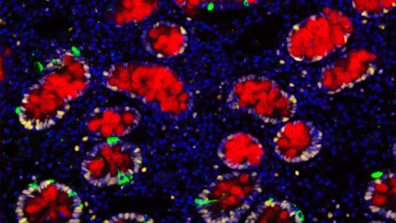
Winton Group
Stem cell biology of the intestine

Research Summary
We focus on intestinal stem cells and their role in colon cancer. These stem cells can develop into various cell types in the gut lining. Our research aims to identify these stem cells and understand how they might cause tumours. We examine their division frequency, the range of cell types they can become, and their local environment. We then study these characteristics in cancer to see how they are maintained in cancerous cells and how these cells respond to treatments. Our goal is to find differences between normal and cancerous stem cells to develop new treatments.
Introduction
Cancers of the colon, like other cancers, arise from the abnormal growth of cells containing oncogenic mutations. This provides the motivation to understand in detail the molecular changes accompanying such mutations. In contrast the impact of mutations on cell fates within affected tissues receives relatively little attention. Yet cancers are comprised of expanded clones of cells. Every mutated clone has a natural history involving discrete steps that establishes the fate of the founding cell and of its clonal descendants to dictate their contribution to, and availability for, neoplastic transformation.
We investigate the origin of clones in the intestinal epithelium to understand the probability of their survival and expansion at each stage and how this varies with different mutations. By defining when during their natural history clones predisposed to cancer become permanently fixed and how much they subsequently grow we can tailor interventions appropriately: aiming either to promote their extinction or limit their growth.

Prof Doug Winton
Senior Group Leader
Focus areas
Related News
See all news-
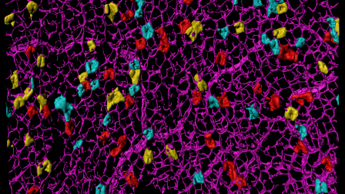
Order of cancer-driving mutations affects the chance of tumour development
3rd December 2025
New research from the Winton Group has revealed that the order of cancer-driving mutations plays an important role in whether tumours in the intestine can develop.
Find out more -

Institute Group Leaders awarded promotions by the University
26th June 2020
We are delighted to announce that several of our Group Leaders have been promoted in the University of Cambridge Senior Academic Promotions.
Find out more -

Institute scientists to work on £20 million “Grand Challenge”
23rd January 2019
Scientists at the Cancer Research UK Cambridge Institute are set to play a key role in a multimillion pound Cancer Research UK project which aims to revolutionise the prevention, diagnosis and treatment of cancer.
Find out more
Further reading
-
STORMing Cancer
Find out moreWe are part of Cancer Grand Challenge team aiming to decipher how inflammation affects the connective tissue that surrounds organs.
-
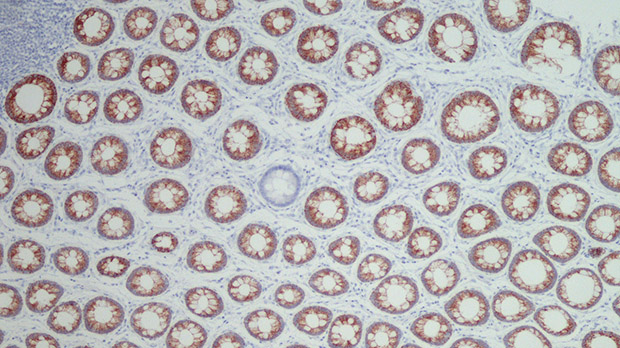
Digging for clues on how bowel cancer starts
Find out moreFormer PhD student Cora Olpe writes about her research to uncover how bowel cancer develops.
Laboratory Efficiency Assessment Framework (LEAF)
The Winton Group contributed to the Institute’s LEAF Silver accreditation, see the Sustainability webpage for more information.